Did you know that on average, U.S. consumers use 6 to 12 cosmetic products daily, containing approximately 200 chemicals? That’s a lot of potential exposure!
With such widespread use, it’s crucial that cosmetic labels provide accurate information to protect consumer health. One wrong ingredient or missing warning could lead to serious consequences like:
- Allergic reactions
- Skin irritations
- Potential long-term health effects
These aren’t just hypothetical scenarios – they’re real concerns backed by FDA reports and scientific studies. That’s why the FDA has established strict guidelines for cosmetic labeling. Let’s dive into these regulations to ensure your products are compliant and consumer-safe!
Table of Contents
What are FDA cosmetic labeling guidelines?
FDA Cosmetic Labeling Guidelines are a set of regulations that dictate how cosmetic products should be labeled in the United States. These guidelines ensure that consumers have access to important information about the products they’re using.
Key aspects of FDA Cosmetic Labeling Guidelines include:
1. Principal Display Panel (PDP)
This is the part of the label that consumers see first. It must include:
- Product Identity: Clearly state what the product is (e.g., “shampoo,” “lipstick”)
- Net Contents: Display the amount of product (e.g., “4 fl. oz.”)
2. Information Panel
This panel should include:
- Name and Place of Business: Your company’s name and address
- Ingredient List: All ingredients in descending order of predominance
- Any required warning statements
3. Ingredient Declaration
- List ingredients in descending order of predominance
- Use INCI (International Nomenclature of Cosmetic Ingredients) names
- Font size must be at least 1/16 inch in height (1/32 inch for small packages)
4. Warning Statements
Certain products require specific warnings. For example:
- Aerosols: “Warning – Avoid spraying in eyes. Contents under pressure. Do not puncture or incinerate.”
- Feminine Deodorant Sprays: “Caution – For external use only. Spray at least 8 inches from the skin.”
Understanding Cosmetics: What Falls Under FDA Regulation?
When it comes to cosmetic labeling, understanding what products actually fall under FDA regulation is important.
According to the Federal Food, Drug, and Cosmetic Act (FD&C Act), cosmetics are defined as “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body…for cleansing, beautifying, promoting attractiveness, or altering the appearance.” This broad definition includes a wide range of products, including:
- Skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial makeup
- Shampoos, permanent waves, hair colors, and deodorants
- Any substance intended for use as a component of a cosmetic product
However, it’s important to note that some products can be both cosmetic and a drug. For example, anti-dandruff shampoos and antiperspirant deodorants fall into both categories. These products must comply with regulations for both cosmetics and drugs.
Interestingly, not all personal care products are regulated as cosmetics. Soaps whose sole cleaning agent is alkali salts of fatty acids fall under the jurisdiction of the Consumer Product Safety Commission, not the FDA. But if any scent or moisturizing agents are added, it becomes a cosmetic.
For organic products, claims are regulated by the USDA’s National Organic Program (NOP), adding another layer of compliance to consider.
If you’re unsure whether your product needs to follow cosmetic regulations, the FDA provides an in-depth guide on product categories. It’s always better to err on the side of caution when it comes to regulatory compliance.
Why do you need to comply with FDA cosmetic labeling guidelines?

Complying with FDA Cosmetic Labeling Guidelines isn’t just a good practice – it’s a legal requirement with far-reaching implications. Let’s dive deeper into why compliance is must:
- To fulfill legal requirements:
FDA Cosmetic Labeling Guidelines are federal regulations. Violating these rules isn’t just a minor oversight – it’s breaking the law. Non-compliance can result in serious consequences:
- Product recalls: The FDA can mandate the removal of non-compliant products from the market.
- Hefty fines: Violations can lead to significant financial penalties.
- Legal action: The FDA may issue warning letters, seek injunctions, or even seize products.
In recent years, we’ve seen the FDA take action against companies for seemingly minor labeling issues, such as marketing claims that inadvertently classified cosmetic products as unapproved new drugs. These cases serve as a stark reminder of how seriously the FDA takes labeling compliance.
- To protect your brand:
Your brand is more than just a logo – it’s the trust consumers place in your products. Non-compliant labeling can severely damage this trust:
- Loss of consumer confidence: Mislabeled products can make consumers question your brand’s integrity.
- Reputation damage: In today’s connected world, news of labeling violations can spread quickly, tarnishing your brand image.
- Potential lawsuits: Incorrect labeling can expose your company to product liability claims.
The cosmetics industry has seen how labeling issues can snowball into major PR crises. From “natural” claims on products containing synthetic ingredients to allergens not properly disclosed, these incidents have led to lawsuits, settlements, and long-lasting reputation damage.
- To ensure consumer safety:
At its core, proper labeling is about protecting your customers. Clear, accurate labels help consumers:
- Make informed decisions: Detailed ingredient lists allow users to avoid allergens or unwanted substances.
- Use products safely: Warning statements and usage instructions prevent misuse and potential harm.
- Identify genuine products: Proper labeling helps consumers distinguish your products from potentially harmful counterfeits.
The cosmetics industry has faced safety issues related to undisclosed ingredients, like the discovery of asbestos in certain talc-based products. Such incidents underscore the critical role that accurate labeling plays in consumer safety.
- To maintain market access:
Compliance isn’t just about avoiding penalties – it’s about keeping your products on shelves:
- Retail partnerships: Many retailers require proof of FDA compliance before they’ll stock your products.
- International trade: Compliance with U.S. regulations is often a prerequisite for exporting cosmetics.
- E-commerce platforms: Online marketplaces increasingly require sellers to comply with local regulations.
We’ve seen how regulatory challenges can impact even large, established brands. Some companies have had to exit entire markets due to difficulties with local regulatory compliance, highlighting how crucial these guidelines are for maintaining market access.
- To avoid costly corrections and recalls:
Prevention is always better (and cheaper) than cure. Compliance helps you avoid:
- Expensive relabeling processes
- Costs associated with product recalls
- Lost revenue from unsellable inventory
The financial impact of a recall can be staggering, not to mention the logistical nightmare of removing products from the market. By prioritizing compliance from the start, you safeguard your business against these potential setbacks.
- To stay ahead of evolving regulations:
The regulatory landscape is always changing. Staying compliant helps you:
- Anticipate future regulatory changes
- Adapt quickly to new requirements
- Demonstrate industry leadership
With the recent passing of the Modernization of Cosmetics Regulation Act (MoCRA), we’re seeing how quickly the regulatory landscape can shift. Companies that prioritize compliance are better positioned to adapt to these changes seamlessly.
By making FDA cosmetic labeling guidelines a priority, you’re not just ticking a box – you’re building a foundation of trust and safety that benefits both your business and your customers. It’s an investment in your brand’s future, ensuring you’re well-positioned to thrive in an increasingly regulated industry.
What are the key provisions of FDA cosmetic labeling guidelines?
FDA Cosmetic Labeling Guidelines are comprehensive regulations that ensure cosmetic products are labeled accurately and safely. Let’s break down the main requirements:
1. Principal Display Panel (PDP)
The PDP is the part of the label most likely to be seen by consumers when displayed for sale. It must include:
- Product identity (what the product is)
- Net quantity of contents
Key aspects:
- Must be large enough to accommodate all required information Information should be prominent and easy to read
- Ensures consumers can quickly identify the product and its quantity
- Must clearly show the product identity and net quantity of contents.
2. Information Panel
This panel typically appears to the right of the PDP (when looking at the front of the package). And as per FDA guidelines, it should include:
- The name and place of business of the manufacturer, packer, or distributor
- The ingredient list is also typically located here, providing transparency about what’s in the product
Key aspects:
- Usually located to the right of the PDP
- Must be large enough to accommodate all required information
- Provides transparency about the manufacturer and product contents
3. Ingredient Declaration
This helps consumers identify potential allergens and make informed choices about the products they use. More details to consider here is,
- Ingredients must be listed in descending order of predominance.
- INCI (International Nomenclature of Cosmetic Ingredients) names should be used.
- Use the names established by FDA regulations or standard references
Key aspects:
- Font size must be at least 1/16 inch in height (1/32 inch for small packages)
- ‘Fragrance’ or ‘Flavor’ can be listed as such without specifying individual components
4. Warning Statements
According to FDA’s cosmetic guidelines, certain products require specific warnings. For example:
Aerosol products: Must warn about avoiding eye contact and not puncturing the container Feminine deodorant sprays: Must caution about external use only and spraying distance
Key aspects:
- Must be prominently displayed and easy to read
- Ensures consumer safety and proper product usage
5. Font Size and Legibility Requirements
The FDA specifies minimum font sizes for different parts of the label to make sure that all required information is easily readable by consumers. Here’s a detailed breakdown of the requirements:
- Minimum font size: Generally, the text on cosmetic labels must be at least 1/16 inch in height. This applies to most of the required information, including the ingredient list.
- Small package exception: For packages with a total surface area available for labeling of less than 12 square inches, the FDA allows a smaller font size of 1/32 inch in height.
- Prominence and conspicuousness: All required label information must be prominent and conspicuous. This means it should be placed on the label with such conspicuousness as to render it likely to be read and understood by ordinary individuals under customary conditions of purchase and use.
- Contrast: The text must have sufficient contrast with the background to make it easy to read. Avoid using similar colors for text and background that could make the information hard to discern.
- Letter height measurement: The FDA specifies that letter height is determined by the lower case letter ‘o’ or its equivalent when upper and lower case letters are used.
- Principal Display Panel (PDP) requirements: The statement of identity and net quantity of contents on the PDP must be in lines generally parallel to the base on which the package rests as it is designed to be displayed.
- Information panel layout: Information required to appear on the information panel should be placed together, without any intervening material, in lines generally parallel to the base on which the package rests.
- Warning statement specifications: Any required warning statements must appear in bold type and in letters at least 1/16 inch in height, unless otherwise specified by regulations.
6. Prohibition of false or misleading claims
Labels cannot make claims that are false, misleading, or unsubstantiated This protects consumers from deceptive marketing practices
By adhering to these key provisions, cosmetic companies not only comply with legal requirements but also demonstrate their commitment to consumer safety and transparency. These guidelines create a standardized labeling system that provides consumers with clear, accurate, and important information about the cosmetic products they’re purchasing and using.
Remember, proper labeling isn’t just about following rules – it’s about building trust with your customers and ensuring their safety. In the next section, we’ll explore why compliance with these guidelines is so crucial for your business
Is your cosmetic brand FDA-compliant?
Complying to these cosmetic regulations is a time-consuming task that can take up to 1-6 weeks for each label. Not just time-consuming, it’s complex, expensive and requires lots of human attention and work.
So if you need to streamline the process and cut down the label review time MAJORLY—we recommend using GoVisually.
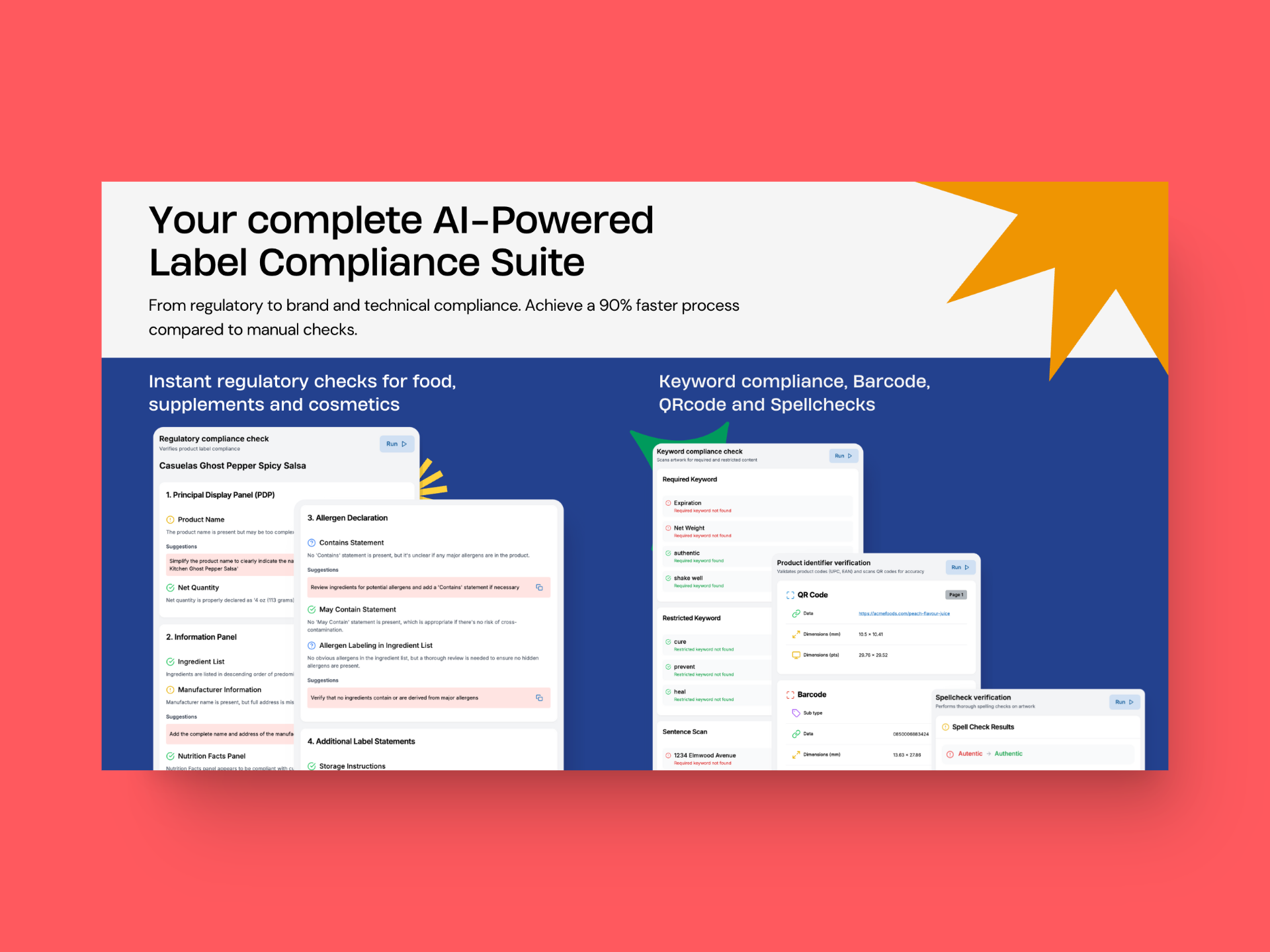
You can easily integrate GoVisually’s compliance AI into your existing workflow and
- Verify ingredient lists with 99.9% accuracy
- Validate marketing claims across different markets
- Reduce recall risks by minimizing label errors
Wondering how?
GoVisually’s compliance AI is developed after rigorous research of a year and back and forth consultations with experts in the field. It offers some of the most advanced features in the market that can boost your label review time significantly, and save you time and money. Here’s how it works:
1. Slash down label review time in half:
GoVisually’s one-click analysis scans labels in seconds, performing instant checks against FDA, MoCRA, and global regulations with 99.99% accuracy. This cuts review times from 2-6 weeks to minutes per label, reducing recall risks and saving costs.
The system’s efficiency leads to a 90% faster review process, enabling your team to focus more on innovation than compliance.
2. Launch product 50% faster:
GoVisually’s customizable playbooks adapt to your product lines and markets, updating in real-time as regulations evolve. With proactive issue flagging and suggested corrections, you’ll see 60% fewer revision cycles and a 50% faster time-to-market.
GoVisually’s multi-faceted checks cover over 50 compliance aspects per label, ensuring thorough reviews without sacrificing speed.
3. Protect your brand:
Ensure flawless compliance and build consumer trust. Our system verifies ingredient lists with 99.9% accuracy, checks INCI naming and ingredient order, and validates marketing claims against FDA and other relevant standards. We also verify required warnings and font size compliance. This detailed approach results in consistent, compliant labeling that enhances your brand’s reputation for transparency and reliability.
4. Improve your overall label review process:
Integrate GoVisually into your workflow with connections to over 3000 apps, including Adobe Creative Suite and others. Our version control and real-time collaboration features reduce communication delays by up to 70%. Detailed compliance summaries keep stakeholders informed, while the system learns from each review. The result is a streamlined process that turns compliance into a competitive advantage in the cosmetics market.
Our compliance AI is here to assist not to replace! It’s a guidance tool to help humans and not replace them, so rely on it accordingly. And if these features look useful for your label review process, try out our FREE label compliance Scorecard tool and boost the efficiency of your label review team.
What’s next for cosmetic labeling?
The FDA continually updates its regulations to ensure consumer safety. Here are some upcoming changes to watch:
- Modernization of Cosmetics Regulation Act (MoCRA): This new act, passed in December 2022, gives the FDA more authority over cosmetics regulation.
- Good Manufacturing Practices (GMP): The FDA is set to propose new GMP regulations by December 29, 2024.
- PFAS Risk Assessment: A report on the risks of PFAS in cosmetic products is due by December 29, 2025.
Remember, proper labeling isn’t just about following rules – it’s about building trust with your customers and ensuring their safety. With GoVisually, you can make the labeling process smoother, faster, and more accurate.
Disclaimer: While our AI compliance checker is highly accurate, it should be used as a tool to aid human expertise, not replace it. Always consult with qualified professionals for final compliance verification.




