Disclaimer: While GoVisually strives to convey accurate information, please refer to the official document offered by the FDA for the most up-to-date guidelines on MoCRA guidelines.
According to the National Center for Health Research, on an average U.S. consumers use 6 to 12 cosmetic products daily, containing approx. 200 chemicals.
Considering its daily usage, 200 chemicals are a lot!
One wrong chemical can lead severe long-term health impacts like;
- Hormonal imbalances
- Reproductive issues
- Risk of cancer
- Risk of Anaphylaxis (a severe life-threatening allergic reaction)
These alarming claims aren’t just speculation – they’re backed by years of rigorous scientific research and documented cases. We found out about these adverse impacts in one of the reports published by the National Library of Medicine. This report clearly claimed how continuous exposure to phthalates (an industrial chemical) can lead to significant reproductive and developmental issues.
Considering these serious and scientifically backed health concerns, the U.S. passed the Modernization of Cosmetics Regulation Act (MoCRA) in December 2022, which was then signed into law. This landmark legislation gives the FDA new authority to regulate cosmetics more effectively.
We understand that complying with MoCRA regulations can be overwhelming and complex. That’s why we have covered all important MoCRA-related details here in digestible bits. Keep reading our ultimate MoCRA guidelines and regulations guide to understand the act completely!
Table of Contents
What is MoCRA?

MoCRA stands for Modernization of Cosmetics Regulation Act of 2022. The introduction of MoCRA as a federal law was one of the most significant updates made by the FDA’s (Food & Drug Administration) regulatory authorities. It expanded FDA’s control over the cosmetic industry and introduced more strict laws to ensure consumer safety.
Additionally, Section 607(a) of the FDCA mandates that facility owners or operators register any site involved in manufacturing or processing cosmetics for U.S. distribution. In contrast, Section 607(c) requires them to list all products made for sale in the U.S.
This act applies to all cosmetic products and their ingredients manufactured, packaged, or distributed for sale in the United States.
MoCRA was enacted to;
- Enhance consumer safety by improving oversight of cosmetic products
- Increase transparency in the cosmetics industry
- Establish new requirements for cosmetic manufacturers and distributors
- Modernize and strengthen the FDA’s ability to regulate cosmetics
- Bring cosmetics regulation more in line with other FDA-regulated products
Why do you need to comply with MoCRA?
MoCRA has drastically changed the process of cosmetic production in the U.S. now. The system has transformed previously voluntary practices into mandatory ones like following Good Manufacturing Practices (GMPs), product registration, etc., The main goal behind this law is to safeguard public health through tighter regulations on cosmetics manufacturers and processes.
That’s why complying with MoCRA is not an option anymore— it’s a must!
Here are some reason as why you should comply with MoCRA;
1. To fulfil legal requirements:
MoCRA is now a federal law, making compliance mandatory for all entities involved in the cosmetics supply chain. This includes manufacturers, packagers, distributors, and importers of cosmetic products in the United States.
Failure to comply with MoCRA can result in severe consequences, such as
- Financial penalties
- Immediate withdrawal of products from the market
- FDA-mandated product recalls
2. To protect your brand against backlash:
Non-compliance with MoCRA can severely violate consumer protection rights, leading to significant backlash. This act was designed to ensure consumer safety and transparency about cosmetic ingredients and manufacturing processes to its consumers.
That’s why consumers rely on regulatory frameworks like MoCRA to feel secure in their purchasing decisions
But when a company fails to comply, it betrays consumer trust and potentially puts public health at risk. This violation of the implicit agreement between businesses and consumers – that products sold are safe and as advertised – can trigger a cascade of negative consequences like
- Legal action from consumers
- Causes social media outrage
- FDA enforcement actions
- Loss of retail partnership
- Widespread consumer distrust
- Can lead to chronic health problems in consumers
- Damages brand’s reputation, attracting media scrutiny
That’s why you should always comply with the most updated laws and regulations in the market. Keep reading our MoCRA guide to understand its requirements point by point here.
What are the key provisions of MoCRA?
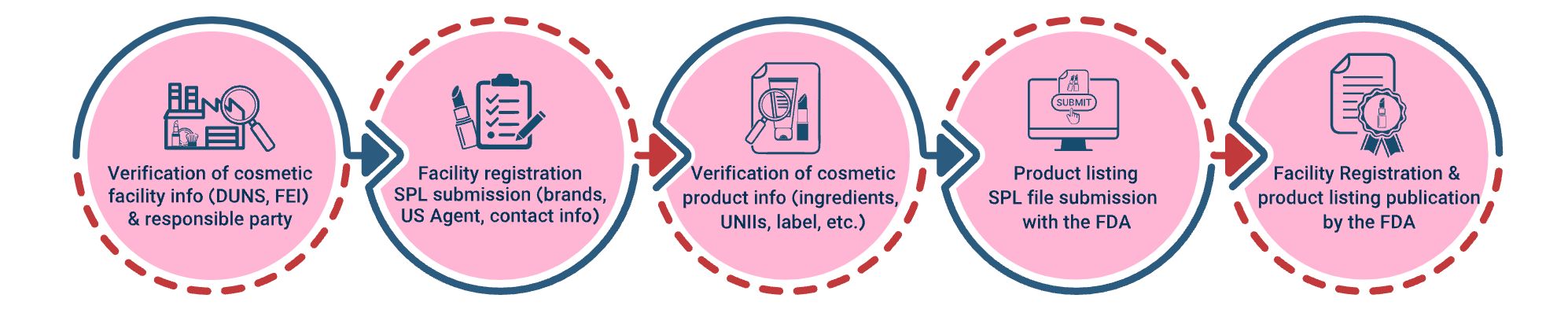
This section includes the key terms cosmetic businesses need to follow under the MoCRA act.
Key Provisions of MoCRA: A Comprehensive Overview
1. Facility Registration
Facility registration under MoCRA requires cosmetic manufacturing and processing facilities to register with the FDA officially. Facility owners or operators manage this biennial process. It helps the government maintain oversight of cosmetic production locations.
Key aspects of the provision:
- Manufacturers and processors must register their facilities with the FDA
- Registration renewal required every two years
- FDA can suspend a facility’s registration under specific circumstances:
- If a product from the facility is likely to cause serious health issues or death
- If the FDA believes other products from the facility may be similarly affected
- If the problem cannot be isolated to one product or is widespread in the facility
- During registration suspension, distributing or selling products from the facility is prohibited. This includes introducing or delivering products into U.S. commerce. Suspension aims to prevent potentially unsafe products from reaching consumers.
2. Product Listing
Product listing mandates that responsible parties – typically manufacturers, packers, or distributors named on the product label – provide detailed information about their cosmetics to the FDA. This transparency allows the FDA to monitor the market better, enabling quicker responses to potential safety issues.
Key aspects of the provision:
- All marketed cosmetic products must be listed with the FDA
- Requires disclosure of product ingredients
- Annual updates necessary to maintain current information
- Applies to both domestic and imported cosmetics
3. Safety Substantiation
According to this provision, a cosmetic manufacturer or entity that introduces a product into interstate commerce must provide evidence supporting the safety of the product. This provision ensures that companies take responsibility for product safety, giving consumers confidence in their purchases.
Key aspects of the provision:
- Companies must ensure and document the safety of their cosmetic products
- FDA doesn’t require specific safety tests for individual products or ingredients
- Responsible persons must keep records supporting adequate safety substantiation
- Manufacturers can use relevant, available safety data to support product safety
- Does not require animal testing, but demands credible safety evidence
- All safety data must be derived from scientific methods
- Records should be readily available for FDA review if requested.
- Companies can choose appropriate methods to substantiate safety as long as they are scientifically sound. For additional guidance, the FDA provides resources on cosmetics safety substantiation
4. Adverse Event Reporting
Adverse event reporting is a crucial component of MoCRA that requires cosmetic companies to inform the FDA about serious health-related incidents associated with the use of their products. These incidents can include
- Death
- Life-threatening experience
- Inpatient hospitalization
- Disability
- Birth defects
- Infections
- Severe disfigurement
Overall, it covers events requiring medical intervention to prevent these outcomes. These events must be reported by responsible persons – manufacturers, packers, or distributors named on the product label. This system helps the FDA identify potential safety concerns quickly, allowing for prompt action to protect consumer health.
Key aspects of the provision:
- Serious adverse events must be reported to the FDA within 15 business days of the company becoming aware of the incident.
- The report content must details of the adverse event, a copy of the product label, and any other relevant information
- Any new information received within a year of the initial report must be submitted to the FDA within 15 business days
- Reports must be submitted using Form 3500A via email or mail. The mail should include complete form, scans of labels and images of the adverse event
- FDA can access and copy certain records related to cosmetic products, including safety records, under specific conditions. This provision supports thorough investigations of safety concerns
- FDA can order a mandatory recall if the investigation agency determines the cosmetic product to be adulterated or misbranded, which can cause serious health risks to the consumer.
5. Good Manufacturing Practices (GMP) Compliance
Good Manufacturing Practices require the FDA to establish regulations for cosmetic facilities, impacting manufacturers and processors directly. These standards ensure consistent product quality and safety, benefiting consumers with reliable products. For businesses, GMPs provide clear guidelines for production processes, potentially reducing liability risks.
GMP is still under discussion however it aims to focus on the below areas;
- GMP will focus on fragrance allergen labeling requirements
- GMP aims to standardized testing methods for detecting and identifying asbestos in talc-containing cosmetic products
MoCRA exemptions for small businesses

MoCra provides certain exemptions for small businesses in their latest guidelines. So, before moving further with the exemptions, let’s understand the FDA’s definition of small businesses.
According to the FDA, “A small business is defined as a business, including its affiliates, whose gross receipts and sales are less than $100 million for the most recent tax year“.
So if your small business meets the FDA’s criteria for small business, then the below exemption should be helpful for you.
Under MoCRA, FDA exempts small businesses from fulfilling
- GMP (Good Manufacturing Practices) compliance
- Facility registration
- Listing requirements
However, these exemptions do not apply if the small business manufacturers or processes include the following cosmetic products:
- Products that regularly come into contact with the mucus membrane of the eye under normal use conditions. This can include items like eye shadows, mascaras, and eye creams
- Any cosmetic products designed to be injected into the body. This category might include certain dermal fillers or cosmetic injectables
- Products intended for internal consumption or use within the body. This could encompass items like ingestible beauty supplements.
- An appearance-altering product that lasts more than 24 hours, under normal usage conditions, and cannot be removed by the consumer. Some of these products include long-lasting hair dyes, tattoo inks, or permanent makeup
Timeline for MoCRA Implementation
| MoCRA Requirements | Current Deadline |
| Safety Substantiation | July 1, 2024 |
| Adverse Events Reporting | July 1, 2024 |
| Product Listing | July 1, 2024 |
| Facility Registration | July 1, 2024 |
| Facility Registration Updates | Within 60 days of any changes to registration information |
| Facility Registration Renewal | Every two years |
| GMP proposal regulations | December 29, 2024 |
| Risk assessment PFAS report | December 29, 2025 |
Note: All the deadlines have been referred from the official government document. These might update or delay as per government instructions, so refer to this doc for most updated news.
Is your cosmetic brand MoCRA-ready? Find out in 2-3 minutes with GoVisually!
Label compliance is an overwhelming and time-consuming process that can keep your teams tied up for up to 2-6 weeks per label. And after the FDA came with MoCRA 2022, label compliance has become a nightmare.
Under the latest MoCRA 2022 act label requirements have significantly changed to include;
- Fragrance Allergen Disclosure: By June 29, 2024, you’ll need to list specific fragrance allergens when they exceed certain concentrations.
- Contact Information: By December 29, 2024, your packaging must include contact details for a US-based representative to handle adverse event reports.
And new regulations mean,
- more revisions
- more stakeholder approvals
- increased time-to-market
- increased label review cost
- and more risk of human errors.
And these above factors make the label review process a vicious and repetitive cycle. That’s why you need a label compliance tool that reviews labels 10 times faster than the manual process.
Compliance A.I. tools like GoVisually’s can
- Reduce review time from 1-6 weeks per label to a few minutes
- Eliminate compliance risk
- Eliminate 1-2% human error into zero
- Achieve 90% faster process compared to manual checks
- Potentially save thousands of dollars per month in review costs
Don’t believe us? Here’s what our customers have to say about GoVisually!

GoVisually’s use cases for cosmetic brands!
GoVisually’s compliance A.I. can transform your time-consuming and manual label compliance process into a streamlined review cycle. It provides some of the most updated and top-notch compliance features in the market, like instant regulatory checks, customizable playbooks, and more. So let’s break down each feature below and help you understand its use cases for your cosmetic brand.
1.Ingredient List Verification
GoVisually’s advanced keyword and claim verification system can automatically check your cosmetic product ingredient lists with 99.9% accuracy. This ensures all ingredients are properly listed and that any restricted substances are flagged immediately. This feature checks label content to determine the following factors in the label:
- Required keywords: As MoCRA requires specific information in the labels, the required keywords feature can verify their presence. This information can include domestic address, fragrance allergen disclosure, facility registration number, etc.
- Restricted keywords: As MoCRA gives the FDA more authority over ingredient safety, this feature can help brand managers monitor prohibited ingredients easily.
- Sentence scan: Sentence scanning can verify that the required domestic address and other such details are formatted correctly and contain the required details.
2. Marketing Claim Validation:
Brand managers can use the customizable A.I. playbook feature to verify that your cosmetic product claims (e.g., “organic,” “natural,“ and “cruelty-free”) comply with regulatory standards across different markets. Product managers can customize playbooks based on
- Regulatory body and industry— GoVisually supports various regulatory bodies across different niches. It includes FDA (food, supplements, cosmetics), U.K. food, CA CFIA food, US CBD hemp, non-GMO, Organic and Gluten-free products, etc.
- Product identifier verification: To verify product codes (UPC, EAN) and Q.R. codes for accuracy
- Proofreading: For typos, spellings, punctuations, and more to bring consistency across all products
- Certification logo match: To check whether the required or claimed certification logos are present on the product label
This feature prevents misleading advertising and potential legal issues for different countries. It also streamlines the process of entering new markets and scaling businesses in no time.
3. Recall Risk Reduction:
GoVisually automated compliance check process with 99.99% accuracy to reduce the risk of label errors. This could lead to costly product recalls, damage brand reputation, and create distrust among the audience.
However if you deploy GoVisually along with your label consultation team, you can significantly minimize label errors within no time.
Disclaimer: While our AI compliance checker is highly accurate, it should be used as tool to aid human expertise, not replace it. Always consult with qualified professionals for final compliance verification.
Future Outlook: What’s next for cosmetic regulation?
After MoCRA, the FDA released a regulation proposal for standardized testing methods, GMP, and a report on PFAS. The deadline for both acts is between December 29, 2024,-2025. So here’s a brief explanation of the future regulations.
1. Asbestos Testing in Talc-Containing Products (December 29, 2023)
A proposal for standardized testing methods to detect asbestos in cosmetic products containing talc.
- Asbestos is a group of naturally occurring minerals that can be carcinogenic when inhaled.
- Talc, a common ingredient in cosmetics, can sometimes be contaminated with asbestos due to their natural co-occurrence.
Through this regulation, FDA aims to ensure consistent and reliable testing methods across the industry to protect consumer safety.
Deadline for Proposal: December 29, 2023
Final Guidance Due: Within 180 days after the proposal (approximately June 2024)
2. Good Manufacturing Practices (GMP) Regulations (December 29, 2024)
A proposal for regulations on Good Manufacturing Practices in the cosmetics industry. GMP are guidelines that ensure products are consistently produced and controlled according to quality standards.
These regulations will help ensure the safety and quality of cosmetic products across the industry.
Deadline for Proposal: December 29, 2024
Final Regulations Due: Before December 29, 2025
3. PFAS Risk Assessment Report (December 29, 2025)
A report assessing the risks of PFAS (Per- and polyfluoroalkyl substances) in cosmetic products. PFAS are a group of man-made chemicals used in various industries, including some cosmetics. Studies have shown that some PFAS can raise health concerns, leading to this risk assessment in cosmetic products.
The report will help determine if further regulations are needed to protect consumer safety.
Deadline: December 29, 2025




